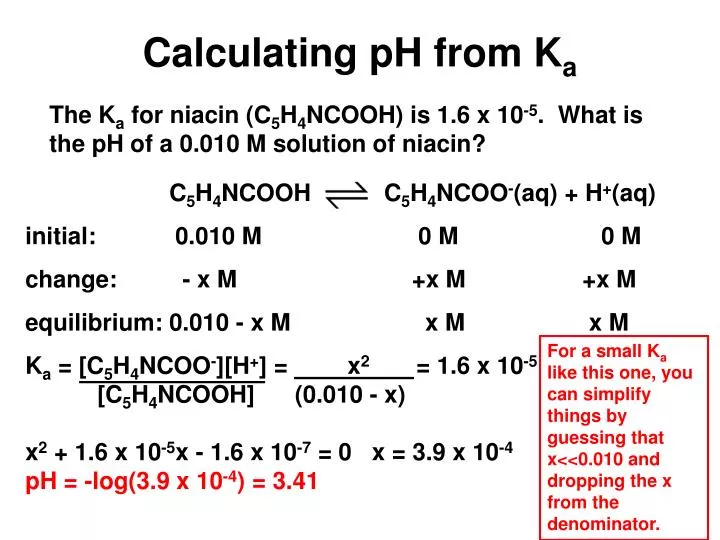
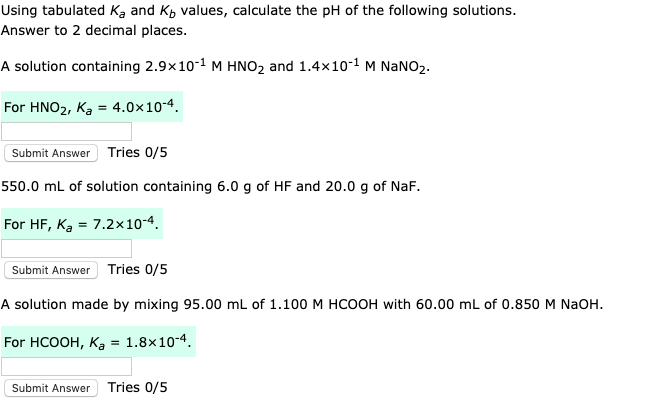
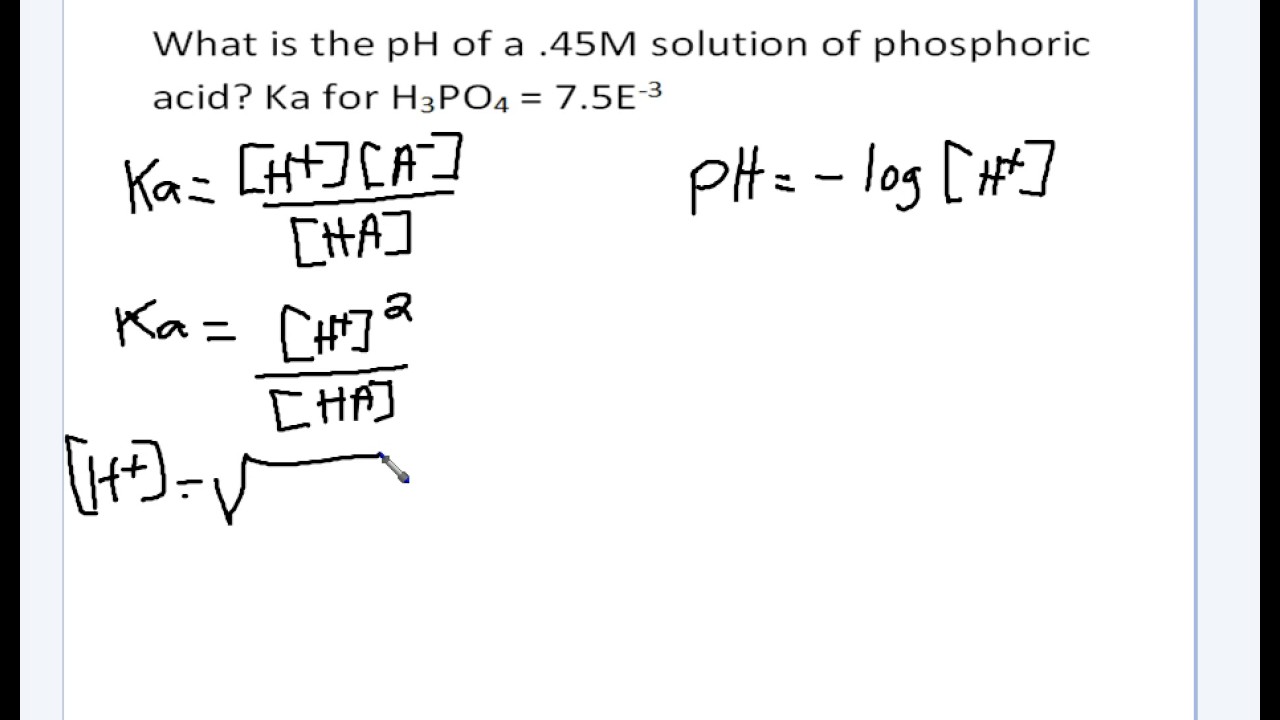
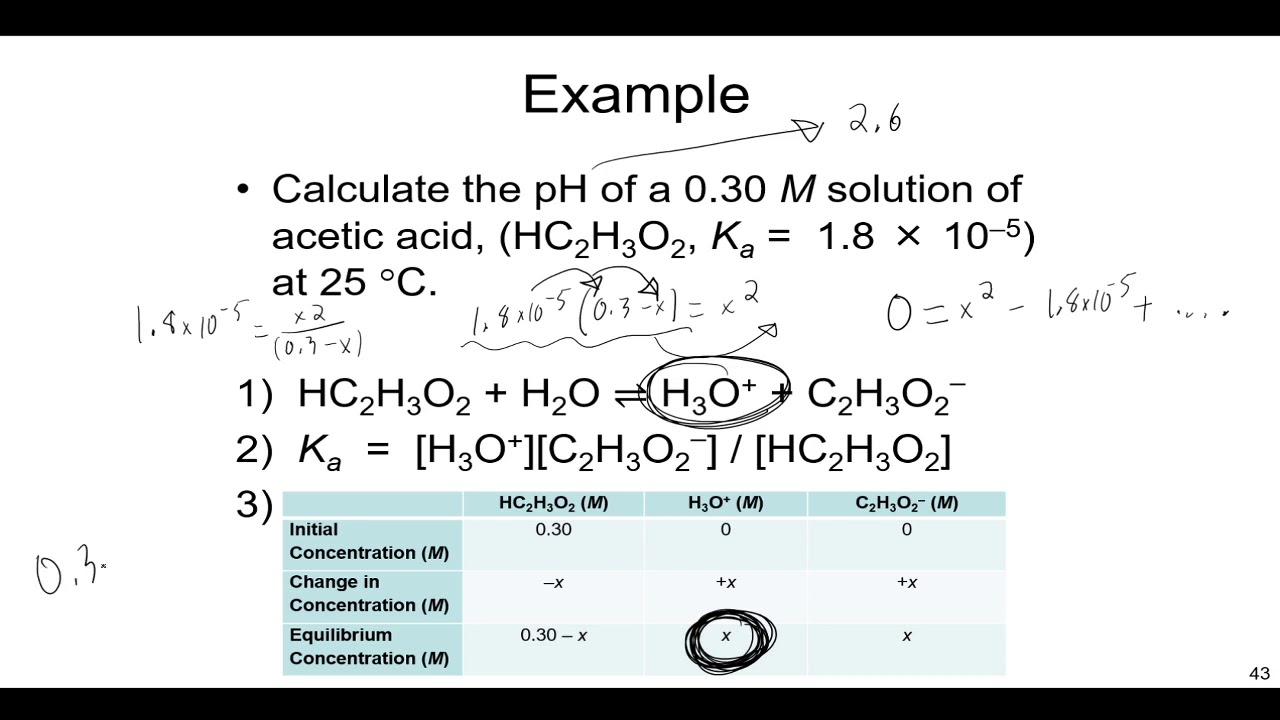
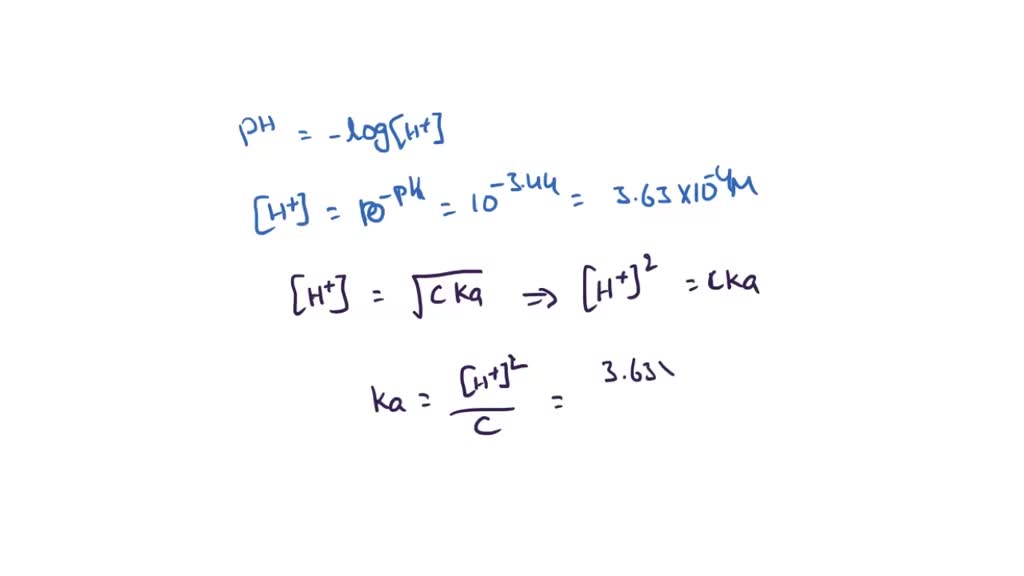pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems - YouTube

SOLVED: Suppose the pH measure of how acidic or basic a water is) value of a soil in a certain area has the following distribution function: k(z2 20z + 100) , 3 <

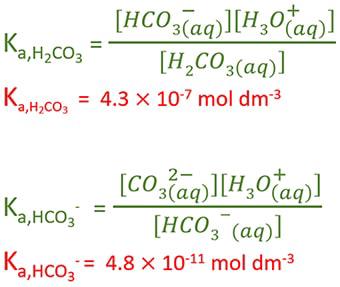
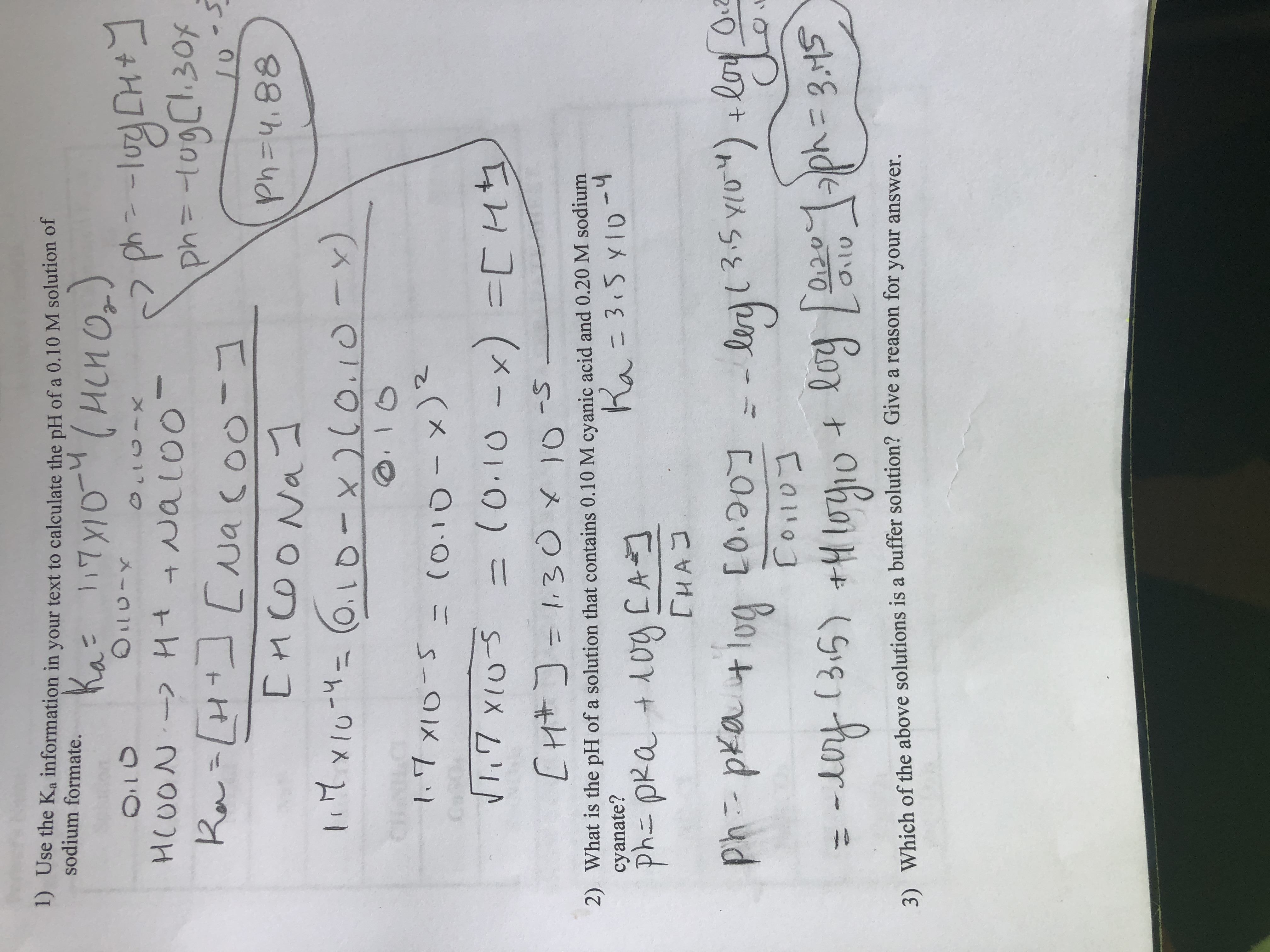
SOLVED: The pH of a solution depends upon the concentration of the acid (or base) as well as the extent of the reaction (2) as given by the equilibrium constant K: The

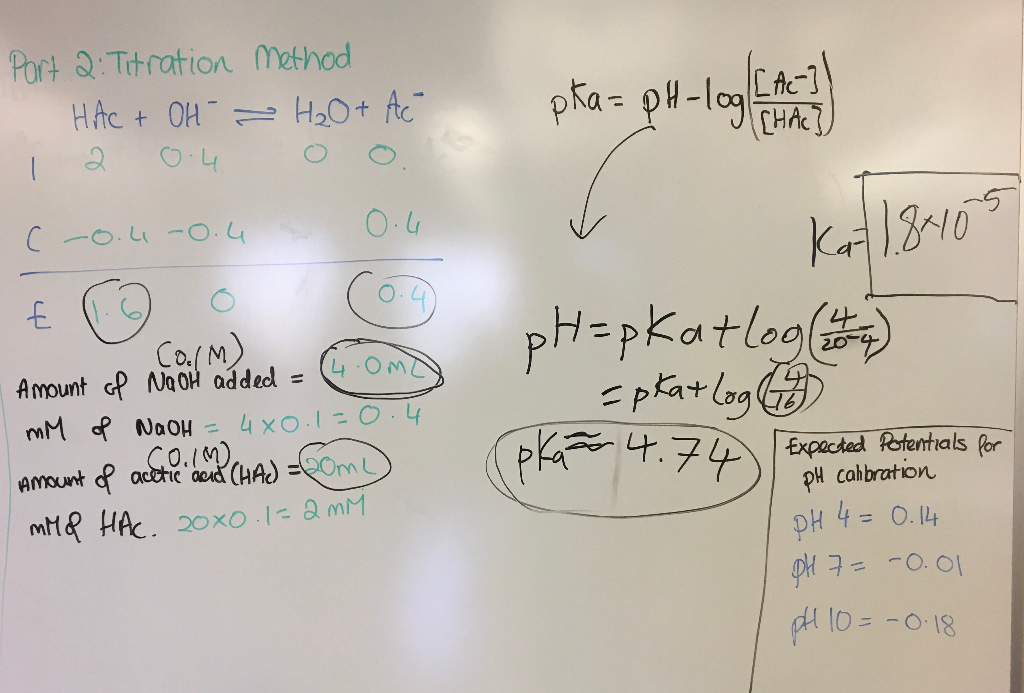
Calculate the pH of the solution formed when 45.0 mL of 0.100 M NaOH is added to 50.0 mL of 0.100 M CH3COOH (Ka = 1.8x10-5). | Homework.Study.com

Acid-Base Buffers Equation & Examples | How to Calculate pH of a Buffer - Video & Lesson Transcript | Study.com